
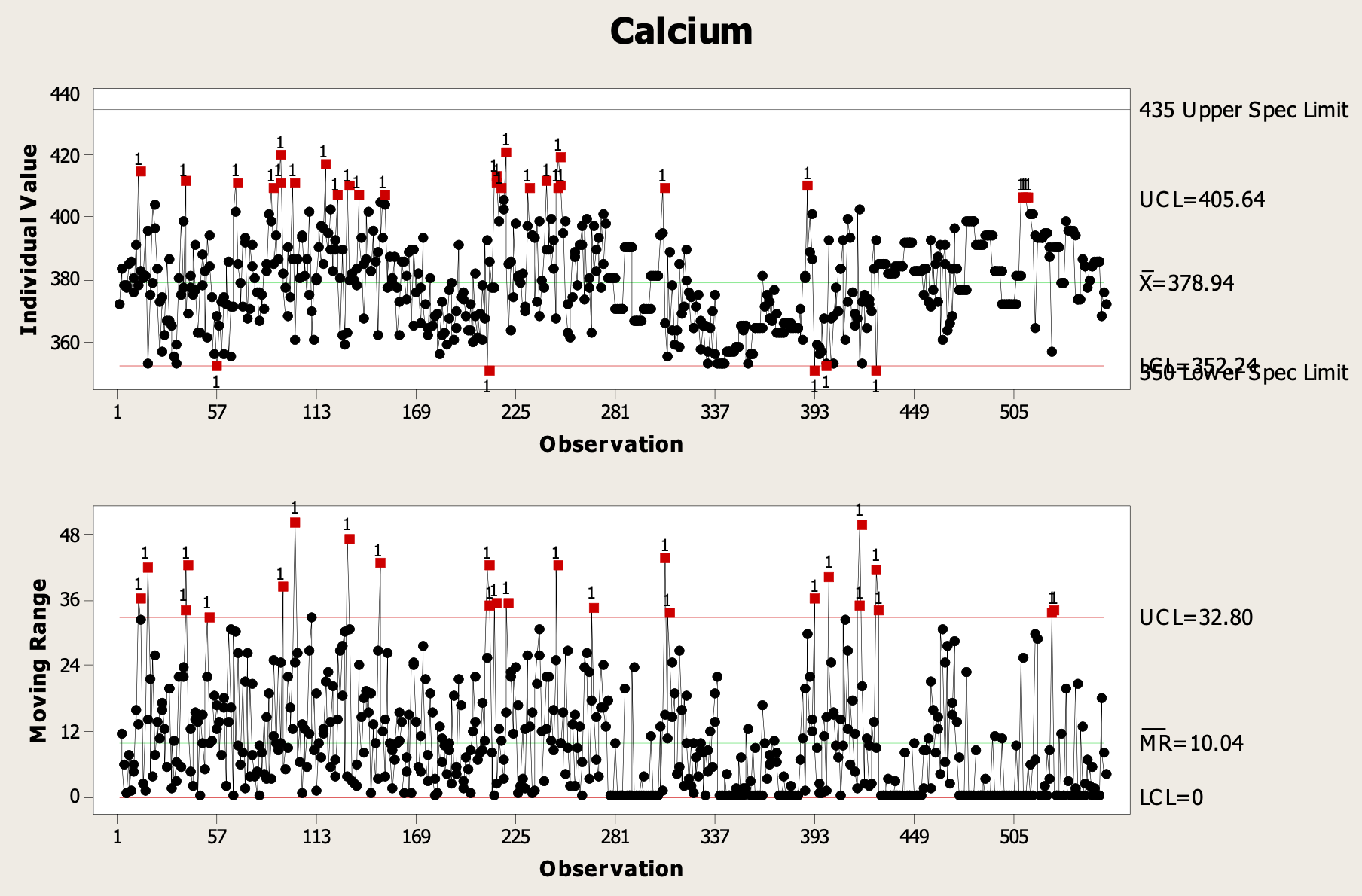
Interpreting Data to Determine Causality
Data can contain much information about a process, and careful diligence is required to recognize and understand anomalies that may be the causes of defects in the process. Investigations often require the use of historical data to find the root cause of a failure or defect, but there can be an almost limitless amount of data out there and searching for causes can be a daunting task. This paper will discuss some of the approaches to analyzing data that can be used to determine causes of out-of-specification (OOS) conditions.
Download your FREE White Paper!
* These fields are required.
FOLLOW PATHWISE!
CHECK OUT OTHER PATHWISE WHITE PAPERS!
