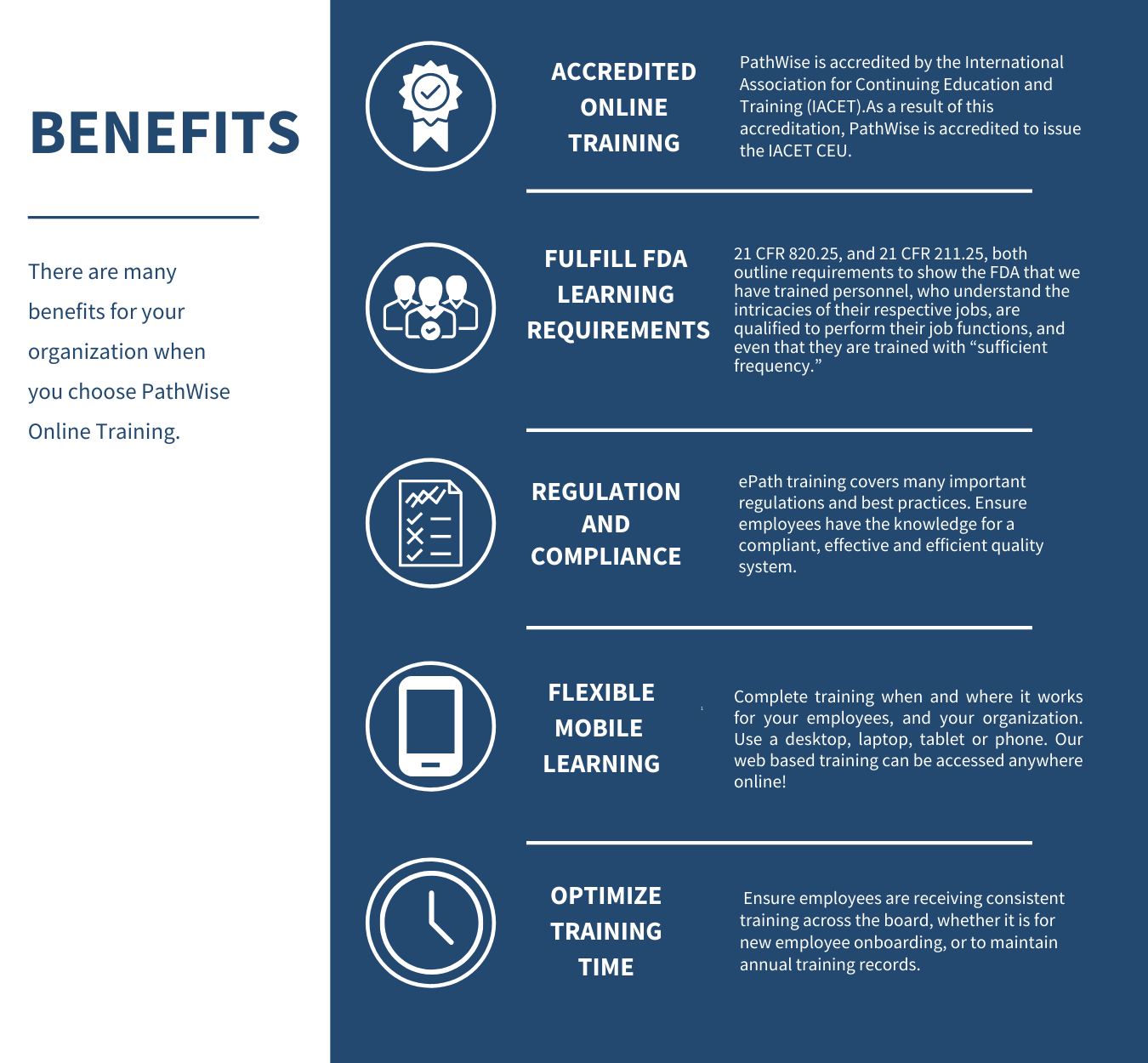

QUESTIONS?
ePath modules can be purchased for individuals, groups, or corporate organizations. For group pricing, and corporate rates, CONTACT US!
* These fields are required.
ePath Log In
Already have access to ePath? Continue your online learning experience!
ONLINE UNIVERSAL LICENSE FOR ONE INDIVIDUAL
ALL Online Training, One Low Price!
$1995/Annually, for one user
- Access 70+ Online Learning Courses
- Earn CEU Credit with every course
- Remain FDA compliant
- Receive up to date training content
- Download a Learning Certificate for every course
Self-paced online e-learning modules include:
On-Demand Webinar titles include: